Oct 14 (Reuters) - The World Health Organization said on Monday it had approved Bavarian Nordic's (BAVA.CO)
, opens new tab mpox vaccine for adolescents aged 12 to 17 years, an age group considered especially vulnerable to outbreaks of the disease that has triggered global concern.
The WHO said in a statement that it gave the Jynneos vaccine prequalification for adolescents on Oct. 8.
The WHO declared mpox a global public health emergency for the second time in two years in August after a new type of the virus spread from the Democratic Republic of Congo to its neighbours.
The United Nations agency approved the use of the vaccine in September as the first shot against mpox in adults, making it easier for badly hit African countries to access the vaccine.
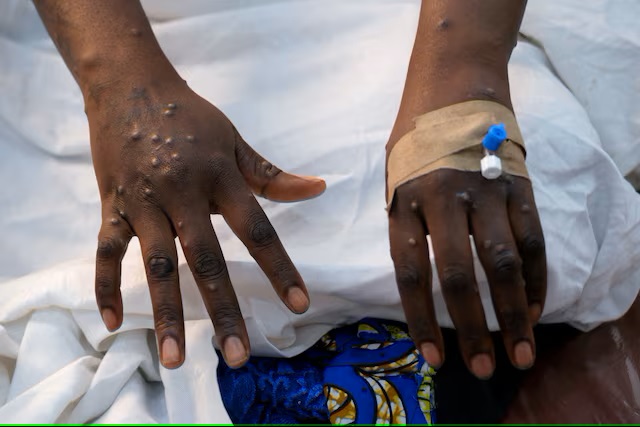
Children, adolescents and those with weakened immune systems have been particularly vulnerable to mpox, a viral infection that typically causes flu-like symptoms and skin lesions filled with pus.